Biotech company CervoMed (CRVO) is on a tear this week as its dementia drug moves forward in clinical trials, bringing it one step closer to potential FDA approval.
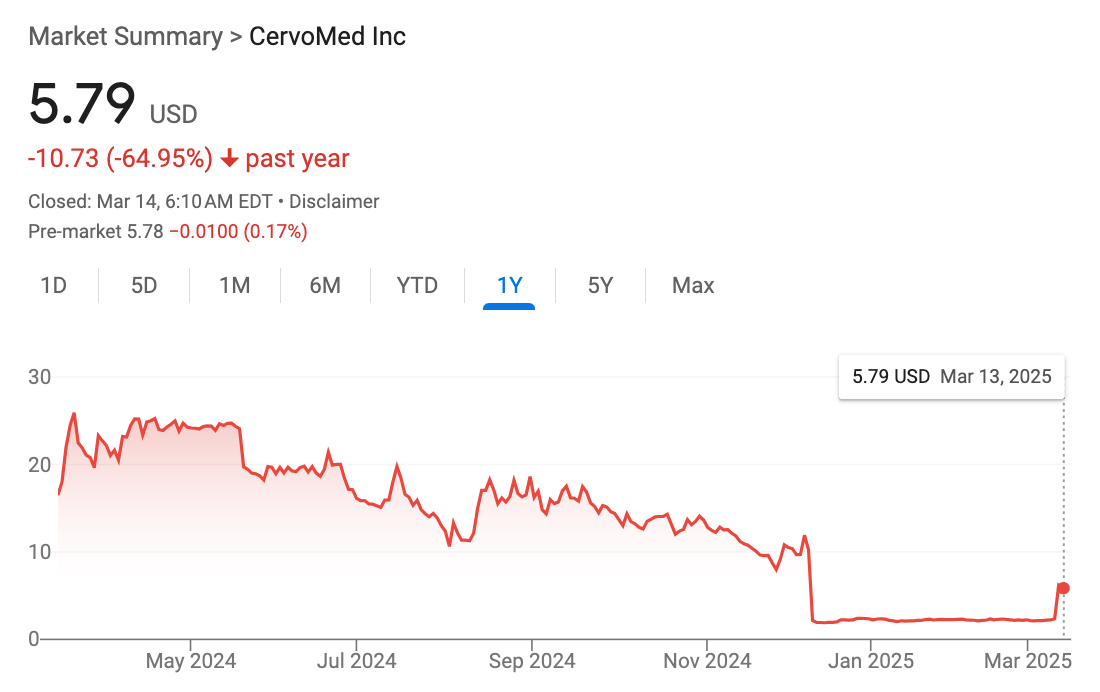
The stock soared 161.44% to $6.2 on Wednesday before retreating to $5.8 yesterday.
A 16-week study of CervoMed’s oral drug, neflamapimod, showed significant improvements in dementia patients’ mobility, particularly in reducing falls. The results could mark a turning point in dementia treatment, according to lead researcher James Glavin.
“There is not a single drug approved in the U.S. for the management of (dementia),” Glavin said in a press release Tuesday.
“My colleagues in the (dementia) clinical community and I look forward to working with CervoMed and regulatory authorities to advance neflamapimod through phase 3 and the regulatory approval process.”
The study focused on Lewy Body Dementia, the third most common neurodegenerative disease after Alzheimer’s and Parkinson’s. Patients in the trial also showed improved cognitive function alongside better mobility.
“It is rare in dementia clinical research to see results with the magnitude of effect and statistical strength as was seen (in this study),” added researcher John-Paul Taylor.
CervoMed now plans to extend the study for another 32 weeks, with final results expected later this year.
Analyst price upgrades drive CervoMed’s rally
Although CervoMed released the study results Monday, the stock rally didn’t take off until Wednesday, after analysts raised their ratings.
Roth MKM nearly doubled its price target to $15 from $7 on Tuesday, giving the stock a “buy” rating.
D. Boral Capital took a more cautious approach, setting a $10 target the same day. Meanwhile, Brookline Capital Management projected CervoMed will post $0.48 earnings per share by Q2 2025. The company last reported a -$0.55 loss per share in Q3 2024.
Wednesday’s surge was the first sign of life for CervoMed since December 2024, when a failed dementia study triggered a brutal 130% single-day drop.
That study also tested neflamapimod but failed to show meaningful results at the 16-week mark — unlike the latest trial, which demonstrated statistically significant improvements.
With an extended study now underway and analysts backing the stock, CervoMed has a shot at redemption. But as with any biotech play, much depends on whether its drug can secure FDA approval.
Your email address will not be published. Required fields are markedmarked